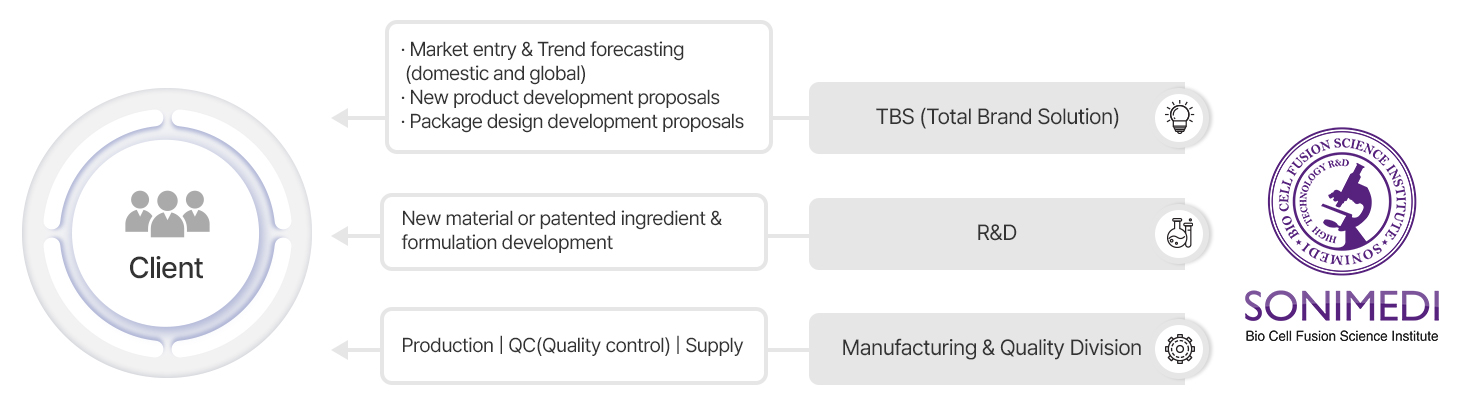
SONIMEDI offers a fully integrated ODM system—covering everything from product planning to production—based on sharp market insights. Working closely with our TBS (Total Brand Solution) team, we deliver customized solutions that reflect client needs and align perfectly with each brand’s identity.
Bespoke ODM Solutions
Bespoke ODM Solutions
End-to-end ODM solutions, tailored to your brand.
Bespoke ODM Strategy
Beyond development—ODM solutions built for your brand and growth.
More than just product development, SONIMEDI delivers tailored ODM solutions that align with each client’s marketing strategy and market positioning. Our approach ensures brand-optimized products and fosters long-term, sustainable growth.

One Client, One Rx
We never use pre-made bulk or semi-finished bases. Every formulation is created in-house from A to Z, making each product uniquely original. We do not share any formula across multiple clients, ensuring the exclusive competitiveness of each brand we serve.

Advanced Bio-Active Materials
We develop and manufacture high-value bio-actives such as peptides, cell-based materials, miRNA, and exosomes. Our ingredients go beyond “concept” marketing — they are clinically functional, scientifically validated, and drive real skin improvement for competitive brand differentiation.

BAT-T | FIU Extraction Technology
We apply BAT-T technology to amplify the efficacy of target ingredients, and FIU, our patented infrared-ultrasonic extraction method (Patent No. 10-1127235), to stably extract bioactive components without degradation. This enables the creation of truly high-performance skincare solutions.

Premium Raw Materials Only
Even for cost-effective products, we never compromise on raw material quality. We do not use low-grade ingredients under any circumstance — our standard is excellence, and quality is our pride.
Strict Quality Control
Superior quality delivers exceptional results
At SONIMEDI, we believe ingredient quality defines product performance.
We use only premium-grade raw materials and apply our patented FIU technology to extract high-purity actives in-house. This ensures safer, more effective formulations with proven results clients can trust.
Raw Material Quality
Inspection & Blending
All formulations are created in-house from scratch. We conduct thorough quality verification based on raw material grade, natural ingredient freshness, and active content stability.
Stability &
Safety Assessment
During the aging period, blended materials are monitored for any physical or chemical changes—such as discoloration, phase separation, or irritation potential—to ensure safety and durability.
In-Process Quality Control (IPQC)
& Hygiene Monitoring
Before filling, semi-finished products undergo secondary QC for key attributes like viscosity, color, and scent. We enforce strict hygiene protocols in our ISO-certified manufacturing environment.
Final Product
Quality Assurance
After filling and packaging, we inspect each unit for defects in containers, labeling accuracy, seal integrity, and overall visual conformity—ensuring the product meets final release standards.
SONIMEDI Company of Headquarters Architectural Image













 Inquiry
Inquiry Business Consulting
Business Consulting





